AMPACe – Accelerometers for Monitoring Physical Activity in Primary Care
Project Number
RG161718
Project Status
Current
Chief investigator
Dr Tejas Kanhere
Team member
Professor Mark Harris
Project Rationale
Physical inactivity is a growing health concern worldwide and has been linked with multiple adverse health conditions including obesity and coronary heart disease. In Australia, more than half of the population do not meet the current recommendations for physical activity (PA). GP’s are in an excellent position to monitor PA in patients, which currently involves use of standardised questionnaires such as the GP Physical Activity Questionnaire (GPPAQ). These can be difficult to administer regularly owing to multiple factors such as lack of software integration. Our study aims to assess if accelerometers, or wrist based activity trackers, can be used in this setting as a potential new way for monitoring PA in patients at risk of physical inactivity.
Project Aim/s
Physical inactivity is a growing concern and is estimated to cause approximately 3.2 million deaths annually[1]. It is associated with raised body mass index (BMI), relative socioeconomic disadvantage and having "poor" self-assessed health[2]. In 2011-2012, an estimated 57% of Australians did not meet the current Australian Physical Activity (PA) recommendations of (150-300 minutes of moderate intensity Physical Activity (PA) or 75-150 minutes of vigorous intensity PA weekly or some combination thereof plus resistance training on >2 days each week[3]). The Royal Australian College of General Practitioners (RACGP) has clinical guidelines on physical activity and its importance as a primary preventative measure including the benefits of using a pedometer for promoting PA[4]. However, no mention is made of accelerometer use in this context.
Our study aims to assess their feasibility in the Australian context by using it as targeted intervention in patients at risk of physical inactivity by their GP, and to see if the intervention can promote PA reliably. Successful completion of this study could provide further guidance on using accelerometers in general practice in Australia as well as provide potential guidelines on safe goal-setting for promoting patient PA using accelerometers.
[1] World Health Organisation 2017: Physical Activity, http://www.who.int/topics/physical_activity/en/
[2] Australian Bureau of Statistics - Australian Health Survey: Physical Activity, 2011-2012, http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4364.0.55.004Chapter1002011-12
[3] Department of Health: Australia's physical activity and sedentary behaviour guidelines 2014, http://www.health.gov.au/internet/main/publishing.nsf/content/health-pubhlth-strateg-phys-act-guidelines
[4] RACGP, Clinical Guidelines: Physical activity (last modified June 2013),
http://www.racgp.org.au/your-practice/guidelines/national-guide/lifestyle/physical-activity/
Project Design and Method
The proposed trial will be a pragmatic randomised trial run across 10 general practices in NSW, with approx. 60 participants recruited overall (6 per practice). Practice and patient recruitment is covered in more detail below and in section 6. Cluster randomisation will be performed with recruited practices randomly allocated into two arms: an intervention arm and a delayed intervention arm. Both the medical centres and the patients will be blinded to which arm they have been assigned to. The study duration is 6 months and is split into two phases lasting three months apiece, A & B, shown graphically below from a participant perspective, with Phase A commencing after the practice has recruited six participants who have completed the consent/initial survey, and Phase B commencing after the 13 week online survey. This design was selected so all participants would have an opportunity to participate while allowing the study to assess maintenance of accelerometer use at 6 months, after the "novelty factor" has worn off.
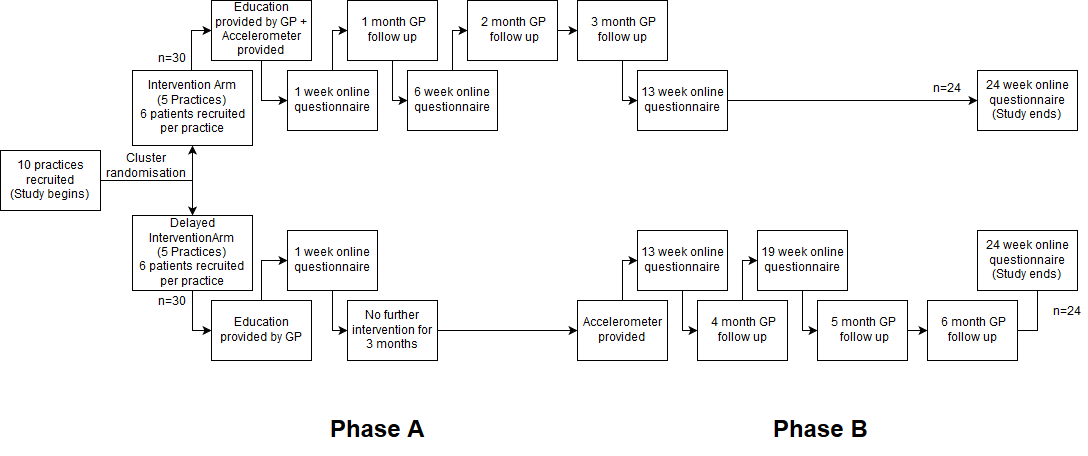
The selected accelerometer for this study is the Xiaomi Mi Band 2 (MB2). At ~$30AUD per device it is readily affordable for most patients. In addition, whilst the Mi Band 2 accuracy is currently being validated in another study, its predecessor the Mi Band 1 was recently recommended as the “best package compared to price” in an accelerometer comparison study[1]. The primary measurements to be assessed from the MB2 will be average daily step count and average time spent physically active. Complex physical activities such as sports will reliably trigger the accelerometer but unfortunately cannot be directly measured.
At the start of the trial GP’s will screen eligible patients for suitability as per the inclusion/exclusion criteria after obtaining verbal consent, and complete the participant data sheet containing anthropometric parameters to give to the patient. This data is not collected by us unless the patient consents to study participation. Patients will then receive a brief educational session on PA by their GP (The GP’s will undergo an active learning module on PA education prior to study commencement). Patients will also be given a participant information sheet together with the completed data sheet and asked to re-present if they have any further questions. If the patient wants to proceed after reading the information sheet they can complete the online consent form + initial survey which includes data entry by the patient from the participant data sheet, completing a GPPAQ and questions on diet and exercise.
The intervention arm will then be mailed the accelerometer and detailed setup/troubleshooting instructions. They will be asked to do a shorter online survey 1 week, 6 weeks, 3 months in to gauge average PA, goals and any behavioural changes, with the information relayed to their GP via email (with practice ID numbers as the only identifier along with average participant data). The participants will also be requested to follow up with their GP’s monthly for three months.
In each monthly follow up sessions GP's will assess how the patient is faring with the accelerometer, and discuss their average daily step count / time spent active compared to the goal set by the participant and the average data across all the patients in the study. Together they will set a new goal for the subsequent visit, with the overall aim at the end of the trial for most participants to achieve 10,000 steps or more daily and increased time spent active. In addition their anthropometric parameters will be measured in each consultation. At the three month mark the intervention arm will see their GP for the last time in the context of the study, and be provided information on maintenance goal setting until the 6-month mark.
Phase B will commence after the 13-week survey. The delayed intervention arm will now also be given an accelerometer. The delayed intervention arm will be asked to follow up monthly thereafter in a fashion identical to the intervention group in Phase A, with the same monitoring and goalsetting suggestions. They will also be asked to complete online survey at the 13, 19 and 24week mark. At the end of the six month period the delayed intervention arm will see their GP for the last time in the context of the study, and all participants will do one last online survey consisting of the GPPAQ, questions on diet, the accelerometer and exercise as well as collecting average daily step count and time spent physically active. These results will then be analysed looking for any behavioural change.
[1] El-Amrawy F, Nounou MI, Are Currently Available Wearable Devices for Activity Tracking and Heart Rate Monitoring Accurate, Precise, and Medically Beneficial?, Healthcare Informations Research, 2015 Oct;21 (4):315-320.
Any Publications
Pending

